Powering Your Plant with Stationary Hydrogen Fuel Cells
By admin | April 8, 2013
Category:
Stationary fuel cells increasingly have been making their way into commercial and institutional facilities. As an environmentally friendly power source, fuel cells are reliable, provide a consistent voltage output, run on various fuels, and produce both electricity and heat. Those advantages have led to stationary fuel cell installations in retail stores, telecommunication facilities, hospitals, and schools.
Far fewer examples exist of stationary fuel cells in manufacturing facilities, although energy experts see potential there as well. Whether or not an industrial facility is a good match depends on public funding and energy costs in a particular region, as well as the way the manufacturing plant uses the energy.
Fuel Cells Gain Traction
Proponents say that stationary fuel cell technology is an under-the-radar option whose time has arrived. Scott Samuelsen, director of the National Fuel Cell Research Center, Irvine, Calif., and co-chair of the California Stationary Fuel Cell Collaborative, Sacramento, Calif., notes that fuel cells are a reliable way to complement the power grid and to provide continuing power to critical circuits if the grid power is disrupted. He said fuel cells can reduce operating costs by providing a way to recover and reuse waste heat. When combined with appropriate power electronics, a fuel cell installation can mitigate voltage sags and harmonics that are harmful to electronic equipment.
Fuel cells produce smaller amounts of greenhouse gases than combustion-based technologies and produce no smog-inducing air pollutants.
Fuel cells are electrochemical devices that produce electricity from hydrogen and oxygen and operate continuously on a steady diet of hydrogen or hydrogen-rich fuel such as natural gas. Fuel cell systems comprise of a front end that conditions the fuel, a back end with the power electronics that connect with the customer or the grid, and the fuel cell module itself.
The fuel cell has an anode (a negative electrode that provides electrons), an electrolyte in the center, and a cathode (a positive electrode that accepts electrons). As hydrogen flows into the anode, a catalyst on the anode helps to separate the gas into protons (hydrogen ions) and electrons (see Figure 1). Individual fuel cells can be combined into a fuel cell stack to increase the total electrical output.
Fuel Cell Types
There are several types of stationary fuel cells, four of which have received significant interest for large stationary power applications. They vary in power output, electrical efficiency, and operating temperatures.
Proton exchange membrane (PEM) fuel cells have a high energy density for their weight and volume. Operating between 50 C and 100 degrees C (122 and 212 degrees F), this fuel cell type starts up quickly and is suitable for load following or for adjusting the power output to meet the power demand in the plant. Terry Howe, manager of solutions engineering for Ballard Power Systems, Burnaby, B.C., Canada, a manufacturer of PEM fuel cells, said the company has targeted chemical companies as a near-term potential stationary application.
Phosphoric acid fuel cells are one of the most mature fuel cells and are among the first to be used commercially. They have a relatively low operating temperature of between 150 and 200 degrees C (302 and 392 degrees F) and are well-suited for load-following applications, according to Bob Tierney, manager of business development and strategic planning for UTC Power, South Windsor, Conn. He said phosphoric acid fuel cells have long stack life—85,000 hours or 10 years of service, up from 40,000 hours five years ago.
Molten carbonate fuel cells operate at 600 to 700 degrees C (1,112 to 1292 degrees F). According to Tony Leo, vice president of applications and OEM engineering at fuel cell manufacturer FuelCell Energy, Danbury, Conn., the high operating temperature of this fuel cell type produces plenty of heat that can be put to other uses in the plant to produce steam, hot water, or chilled water. The high operating temperature also allows for the re-forming process, which extracts hydrogen from natural gas, to take place right in the fuel stack, saving the cost of an external re-former.
Solid oxide fuel cells are the most recent to be commercialized for stationary applications. Historically, solid oxide fuel cells, which operate at up to 1,000 degrees C (1,832 degrees F), have posed significant durability challenges. Yet in February, after eight years of development, Silicon Valley start-up Bloom Energy, located in Sunnyvale, Calif., officially unveiled its Bloom Box solid oxide fuel cell, which the company claims uses low-cost materials and runs on a wider range of fuels than other fuel cell types.
Making Economic Sense
Several factors go into the decision of whether to install a fuel cell, according to Dan Rastler, program manager for the energy storage and distributed generation program at the Electric Power Research Institute, Palo Alto, Calif. Capital costs of fuel cells are relatively high compared to the retail cost of electricity from the grid, he said. In making a decision, manufacturers need to compare the retail rate (wholesale power costs as well as transmission and delivery costs) of power from the electric grid with the cost of purchasing, installing, and running the fuel cell.
The retail rate of electricity from the grid varies widely from region to region, roughly from 7 to 16 cents per kWh. In general, on-site power generation is more attractive in regions where there is a combination of high electricity rates and low natural gas prices.
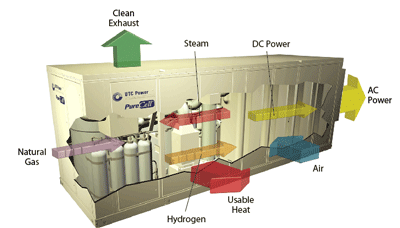 Figure 2In this cutaway of a phosphoric acid fuel cell, the fuel processor re-forms natural gas into hydrogen gas, which is fed into the fuel cell stack. In the stack, hydrogen gas and air are combined in an electrochemical process that produces direct current (DC) power, water, and heat. The byproduct water is used in the operation of a power plant. The byproduct heat is available for meeting other requirements in the facility, such as creating hot water, space heating, or cooling. The DC power provided by the fuel cell stack is conditioned to provide alternating current (AC) power output. Image courtesy of UTC Power, South Windsor, Conn.
Figure 2In this cutaway of a phosphoric acid fuel cell, the fuel processor re-forms natural gas into hydrogen gas, which is fed into the fuel cell stack. In the stack, hydrogen gas and air are combined in an electrochemical process that produces direct current (DC) power, water, and heat. The byproduct water is used in the operation of a power plant. The byproduct heat is available for meeting other requirements in the facility, such as creating hot water, space heating, or cooling. The DC power provided by the fuel cell stack is conditioned to provide alternating current (AC) power output. Image courtesy of UTC Power, South Windsor, Conn. In several states, including Connecticut and California, net metering—a method of crediting customers for electricity that they generate that is in excess of what they purchase from the utility—can make fuel cells attractive economically.
Joel Rinebold, director of energy incentives for the Connecticut Center for Advanced Technology Inc., East Hartford, Conn., said financial incentives exist at both the state and federal levels. The organization will run the numbers for Connecticut companies that are considering installing fuel cells, as well as offer advice on getting the best return on investment. Other states that offer significant financial incentives are New York, New Jersey, and California. On the federal level, opportunities for depreciation and investment tax credits can offset the cost of the fuel installation by 30 percent.
Running a fuel cell as much as possible makes economic sense to pay off the investment sooner. For most types of fuel cells, this practice also reduces wear and tear. “Fuel cells want to run flat out all of the time. They don’t want to cycle up and down like reciprocating engines,” UTC’s Tierney.
Whether a facility can make good use of the electricity and the heat determines whether a it is a good candidate for a fuel cell installation (see Figure 2). The real key in determining whether a fuel cell makes sense is if the plant can make use of the heat that drivesthe reforming process to crack the chemical bonds in the fuel to harvest the hydrogen for the fuel cell, Tierney said (see Real-world Installations).
Those requirements can exist on the process side or to heat the buildings themselves. “Those are questions we draw out with our customers. There is a heat stream, and you can use it for process water, for space heating, for domestic hot water, and for dehumidification,” Tierney said.
Fuel Cells in the Pipeline
David Dornfeld, a professor of mechanical engineering at the University of California, Berkeley, and director of the Laboratory for Manufacturing and Sustainability, finds the idea of using fuel cells to power manufacturing plants intriguing, although he thinks that more research is needed for that to happen. “We need to do some studies for heavy manufacturing, places that do machining, casting, and things that use a lot of power, and what kinds of response times and duty factors you can get from fuel cells,” he said.
Meanwhile, new stationary fuel cell projects have continued apace. In February Ballard signed a memorandum of understanding with K2 Pure Solutions, a chemical producer, for a 1-MW PEM fuel cell at its salt-to-bleach plant in Pittsburg, Calif. The fuel cell will convert byproduct hydrogen generated from salt-to-bleach production into base load electricity that will then be used to power K2’s bleach production operation. The same month Bloom Energy signed an agreement for a 500-kW unit in Coca-Cola Co.’s Odwalla plant in Dinuba, Calif. The fuel cell will run on redirected biogas and provide 30 percent of its power needs.

Side by side, we move metal fabrication forward.
FMA unites thousands of metal fabrication and manufacturing professionals around a common purpose: to shape the future of our industry, and in turn shape the world.
Learn More About FMA



